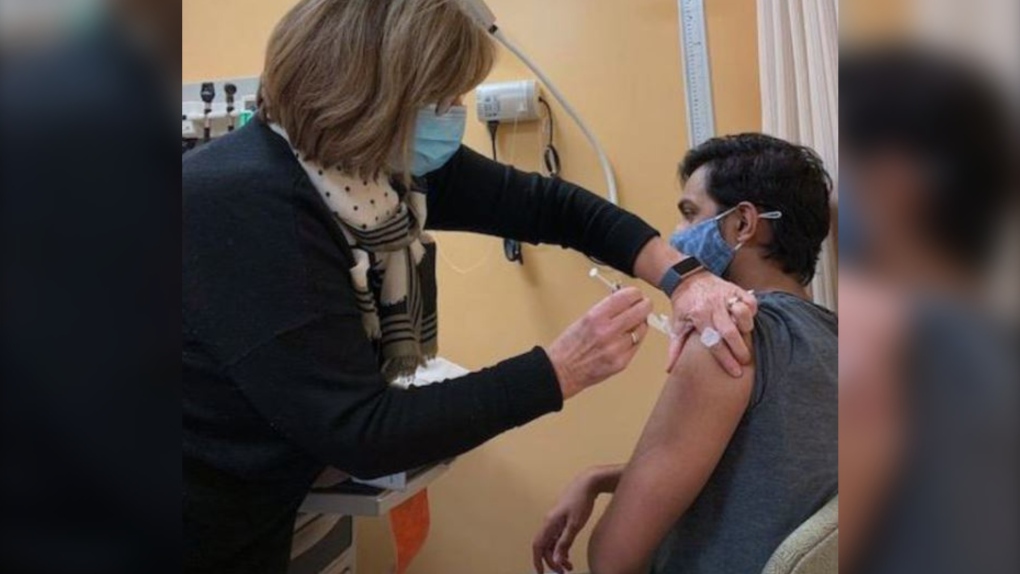
SASKATOON -- A Canadian-made COVID-19 vaccine is now in the arms of volunteers taking part in clinical trials.
Late last year, the University of Saskatchewan's Vaccine and Infectious Disease Organization – International Vaccine Centre (VIDO-InterVac) received Health Canada approval to move into human clinical trials for its vaccine candidate.
According to the university, the first doses of the lab's vaccine candidate were administered to volunteers at the Canadian Center for Vaccinology in Halifax.
In total, 108 volunteers have been selected to participate in a placebo-controlled study at the centre.
The lab's COVAC-2 candidate is a "subunit" vaccine that relies on purified viral proteins and would not require the ultra-cold storage of the mRNA-based Pfizer BioNTech vaccine.
It is created using the same technique as vaccines commonly given to prevent diphtheria and whooping cough.
The vaccine requires two doses, administered 28 days apart.
VIDO-InterVac CEO Dr. Volker Gerdts has previously said he believes the lab's vaccine candidate may offer better protection against variant coronavirus strains.
Earlier this month, Prime Minister Justin Trudeau highlighted efforts underway at VIDO-InterVac to build a vaccine manufacturing facility that could make as many as 40 million doses annually.
The facility, built with a mix of federal and provincial funding, is expected to be up and running by early 2022.